PIPELINE
SLIT PROJECTS
SLIT PROJECTS
Our oral SLIT projects are positioned in the growing market of “immune modulators”.
According to the current analysis of Reports and Data, the global immunomodulators market is expected to reach USD 240 Billion by the year 2026, at a CAGR of 5.4 %.
Immune modulators can be used to regulate the immune response in a variety of ways : for example, they can decrease unwanted inflammatory responses.
Immune modulators can be useful in a wide range of chronic diseases, such as multiple sclerosis, allergy, cancer, and asthma, which is one of the key reasons for the significant growth in this market in recent years.
In this market, our SLIT products will take market share from products that are currently only available as injectable products.
Figure : BioLingus SLIT Technology : the new frontier in patient care > restoring the immune system to achieve long term disease remission
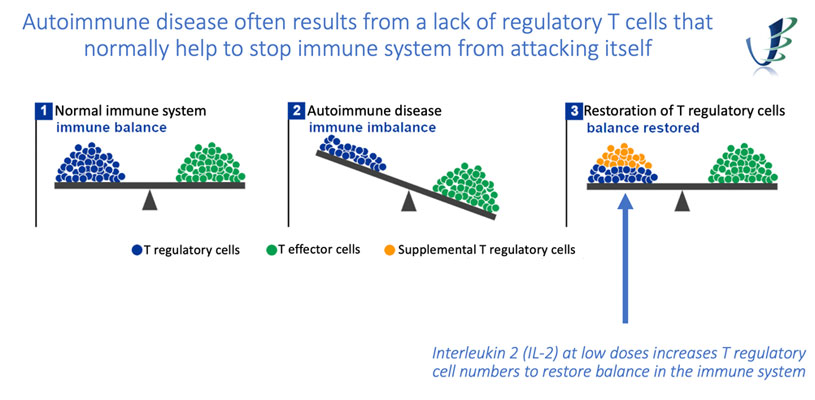
Project : Sublingual IL2 (BL SLIT I)
The aim of this project is to develop low dose IL-2 for treatment of early onset juvenile Type I diabetes. Low dose injectable IL-2 in animal models has been shown to restore the balance between regulatory and effector T cells and, in this way, can help to reverse autoimmunity and the development of type I diabetes.
Looking ahead, we anticipate that having a suitable oral formulation of IL-2 to use in children with recent onset type I diabetes will be important.
Once the proof of concept has been achieved for type I diabetes, we expect that sublingual IL-2 can be further developed for a range of other autoimmune/allergic disorders, all of which are likely to benefit from restoring the balance between regulatory and effector T cell. These diseases include:
- Asthma
- Psoriasis
- Lupus
- Rheumatoid arthritis
- Multiple sclerosis
Project : Sublingual Interferon-alfa (BL SLIT II)
We plan to develop sublingual interferon-alfa for the prevention of viral infections, such as influenza and coronaviruses (such as COVID-19).
The rise in prevalence of viral respiratory infections in humans and increase in health care expenditure are projected to boost the growth of the global respiratory antiviral treatment market.
Respiratory syncytial virus, influenza virus, parainfluenza virus, adenovirus, rhinovirus, and coronavirus belong to the respiratory virus infections group.
Increases in the number of patients with influenza, coronavirus, upper respiratory tract infections (URTI), and pneumonia is anticipated to fuel the growth of the global respiratory antiviral treatment market from 2020 to 2030.
Influenza can cause severe illness or death especially in high-risk patients, such as young children and the elderly. According to the WHO, influenza was estimated to result in about 3-5 million cases of severe illness and about 290,000-650,000 deaths globally in 2018.
Similarly, the Covid-19 pandemic has demonstrated the importance of having preventive treatments for respiratory viruses.
Interferon-alfa has potent anti-viral effects that can not only help to treat viral infection but also prevent them. However, to date, there have been two significant barriers to using IFN as a preventative agent.
The first is that it usually requires parenteral administration (e.g. intravenous injection).
The second is that such treatment is frequently associated with significant systemic side effects that limit their tolerability to patients.
To circumvent these problems, we previously developed a commercial version of IFN-⍺ that is delivered as a sublingual tablet (i.e. under the tongue). This product, which was marketed under the brand name Immulin, was originally used to treat viral hepatitis B and C.
Not only was this treatment highly convenient to administer – since it enabled treatment without the need for injections – it showed a very safe side effect profile with high patient tolerability and compliance.
